Age-related difference changes semen quality and seminal plasma protein patterns of Thai native rooster
Main Article Content
Abstract
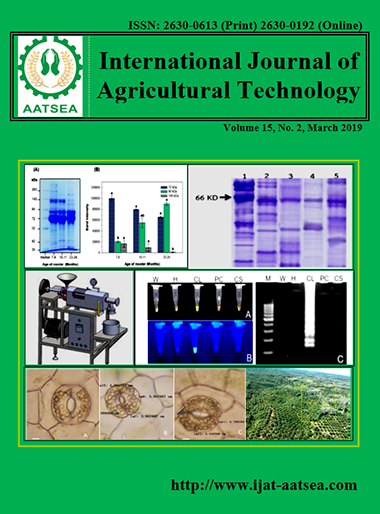
The association between age and semen quality of Thai native roosters (Leung hang khao) in relation to patterns of seminal plasma proteins were determined. Eighteen of the native roosters were grouped according to the ages 7-8, 10-11 and 23-24 months. Semen was collected by abdominal massage and evaluated for different physical parameters. Results showed that semen volume, color, pH and sperm concentration were not significantly different among the different age groups. In contrast, sperm viability was found to be significantly different. Sperm viability was significantly higher in the 7-8 (93.34a ± 2.17) than the 23-24 months age category (71.38b ± 7.44). Seminal plasma proteins were separated using sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). Based on our findings, proteins with molecular weight fractions of 72, 90, 140, 220, and 260 kDa showed patterns of expression in 7-8, 10-11, and 23-24 month old roosters, respectively. Moreover, proteins with molecular weight fractions of 72, 90, and 140 kDa were shown sharply patterns of expression among age of roosters. These results indicate that sperm vitality (percentage of live sperm or dead sperm) and proteins with molecular weight fractions of 90, and 140 kDa are associated with age. Proteins with molecular weight fractions of 90 and 140, kDa may serve as markers to determine semen quality in Thai native roosters.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Al-Aghbari, A., Engel, H. N., Jr. and Froman, D. P. (1992). Analysis of seminal plasma from roosters carrying the Sd (sperm degeneration) allele. Biol Reprod. 47:1059-63.
Almahdi, A., Ondho, Y. and Sutopo, S. (2014). Comparative studies of semen quality on different breeds of chicken in Poultry Breeding Center, Temanggung-Central Java. International Refereed Journal of Engineering and Science. 3:94-103.
Atikuzzaman, M., Sanz, L., Pla, D., Alvarez-Rodriguez, M., Ruber, M., Wright, D., Calvete, J. J. and Rodriguez-Martinez, H. (2017). Selection for higher fertility reflects in the seminal fluid proteome of modern domestic chicken. Comp Biochem Physiol Part D Genomics Proteomics. 21:27-40.
Avital-Cohen, N., Heiblum, R., Argov-Argaman, N., Rosenstrauch, A., Chaiseha, Y., Mobarkey, N. and Rozenboim, I. (2013). Age-related changes in gonadal and serotonergic axes of broiler breeder roosters. Domest Anim Endocrinol. 44:145-50.
Borziak, K., Alvarez-Fernandez, A., T, L. K., Pizzari, T. and Dorus, S. (2016). The Seminal fluid proteome of the polyandrous Red junglefowl offers insights into the molecular basis of fertility, reproductive ageing and domestication. Science Report. 6.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248-54.
Brandon, C. I., Heusner, G. L., Caudle, A. B. and Fayrer-Hosken, R. A. (1999). Two-dimensional polyacrylamide gel electrophoresis of equine seminal plasma proteins and their correlation with fertility. Theriogenology. 52:863-873.
Campbell, R. C., Hancock, J. L. and Rothschild, L. (1953). Counting Live and Dead Bull Spermatozoa. Journal of Experimental Biology. 30:44.
De Souza, F. F., Barreto, C. S. and Lopes, M. D. (2007). Characteristics of seminal plasma proteins and their correlation with canine semen analysis. Theriogenology. 68:100-106.
Douard, V., Hermier, D., Magistrini, M. and Blesbois, E. (2003). Reproductive period affects lipid composition and quality of fresh and stored spermatozoa in Turkeys. Theriogenology. 59:753-764.
Elagib, H. A. A., Musharaf, N. A., Makawi, S. A. and Mohamed, H. E. (2012). The Effects of Age and Season on Semen Characteristics of White Leghorn Cocks under Sudan Conditions. International Journal of Poultry Science. 11:47-49.
Iaffaldano, N., Manchisi, A. and Rosato, M. P. (2008). The preservability of turkey semen quality during liquid storage in relation to strain and age of males. Anim Reprod Sci. 109:266-73.
Karaca, A. G., Parker, H. M. and McDaniel, C. D. (2002). Elevated body temperature directly contributes to heat stress infertility of broiler breeder males. Poult Sci. 81:1892-7.
Killian, G. J., Chapman, D. A. and Rogowski, L. A. (1993). Fertility-associated proteins in Holstein bull seminal plasma. Biol Reprod. 49:1202-7.
Mann, T. (1981). Male reproductive function and semen : themes and trends in physiology, biochemistry, and investigative andrology / Thaddeus Mann, Cecilia Lutwak-Mann, Springer-Verlag, Berlin ; New York.
Marzoni, M., Castillo, A., Sagona, S., Citti, L., Rocchiccioli, S., Romboli, I. and Felicioli, A. (2013). A proteomic approach to identify seminal plasma proteins in roosters (Gallus gallus domesticus). Anim Reprod Sci. 140:216-23.
McDaniel, G. R. and Craig, J. V. (1959). Behavior Traits, Semen Measurements and Fertility of White Leghorn Males. Poultry Science. 38:1005-1014.
Novak, S., Ruiz-Sanchez, A., Dixon, W. T., Foxcroft, G. R. and Dyck, M. K. (2010). Seminal plasma proteins as potential markers of relative fertility in boars. J Androl. 31:188-200.
Pilch, B. and Mann, M. (2006). Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biology. 7: R40.
Rakha, B. A., Ansari, M. S., Akhter, S. and Blesbois, E. (2017). Effect of season and age on Indian red jungle fowl (Gallus gallus murghi) semen characteristics: A 4-year retrospective study. Theriogenology. 99:105-110.
Sarabia Fragoso, J., Pizarro Diaz, M., Abad Moreno, J. C., Casanovas Infesta, P., Rodriguez-Bertos, A. and Barger, K. (2013). Relationships between fertility and some parameters in male broiler breeders (body and testicular weight, histology and immunohistochemistry of testes, spermatogenesis and hormonal levels). Reprod Domest Anim. 48:345-52.
Shanmugam, M., Rajkumar, U., Reddy, M. R. and Rao, S. V. R. (2012). Effect of age on semen quality in naked neck and dwarf chicken under tropical climatic conditions. Animal Production Science. 52:964-968.
Shanmugam, M., Vinoth, A., Rajaravindra, K. S. and Rajkumar, U. (2014). Evaluation of semen quality in roosters of different age during hot climatic condition. Animal Reproduction Science. 145:81-85.
Slowinska, M., Olczak, M., Wojtczak, M., Glogowski, J., Jankowski, J., Watorek, W., Amarowicz, R. and Ciereszko, A. (2008). Isolation, characterization and cDNA sequencing of a Kazal family proteinase inhibitor from seminal plasma of turkey (Meleagris gallopavo). Comp Biochem Physiol B Biochem Mol Biol. 150:207-15.
Sundaram, V., Rajeswari, D., Manian, R. P. and Sridharan, T. B. (2016). Analysis of Seminal Plasma Proteins of South Indian Jersey and Hybrid Bulls and their Correlation with Semen Quality. Asian Journal of Animal Sciences. 10:92-98.
Tabatabaei, S., Chaji, M. and Mohammadab, T. (2010). Correlation Between Age of Rooster and Semen Quality in Iranian Indigenous Broiler Breeder Chickens. Journal of Animal and Veterinary Advances. 9:195-198.
Troedsson, M. H. T., Desvousges, A., Alghamdi, A. S., Dahms, B., Dow, C. A., Hayna, J., Valesco, R., Collahan, P. T., Macpherson, M. L., Pozor, M. and Buhi, W. C. (2005). Components in seminal plasma regulating sperm transport and elimination. Animal Reproduction Science. 89:171-186.
Zhang, X., Berry, W. D., McDaniel, G. R., Roland, D. A., Liu, P., Calvert, C. and Wilhite, R. (1999). Body weight and semen production of broiler breeder males as influenced by crude protein levels and feeding regimens during rearing. Poult Sci. 78:190-6.